When we talk about workplace hazards, it's easy to picture the obvious ones—a slippery floor or a chemical that burns on contact. But some of the most serious threats are completely invisible, working silently over months or years to cause devastating, long-term harm. These are CMR substances: Carcinogenic, Mutagenic, and Reprotoxic chemicals.
Think of them as "stealth threats." They don't announce their presence with a sudden bang or an immediate injury. Instead, exposure to these substances can lead to cancer, cause genetic mutations, or damage reproductive health, with the effects often appearing long after the exposure has stopped. This delayed impact makes them uniquely dangerous and incredibly difficult to manage without a proactive strategy.

A Closer Look at the Three Threats
To really understand the risk, you have to break down the CMR acronym. Each letter represents a distinct, insidious way these substances can attack the human body. Getting a handle on these categories is the first step toward protecting your team.
We can summarise the key differences in a simple table.
CMR Substances at a Glance
| Category | Definition | Primary Health Risk | Common Industrial Examples |
|---|---|---|---|
| Carcinogenic | A substance or mixture that can cause cancer or increase its incidence. | Uncontrolled cell growth (tumours) | Asbestos, benzene, formaldehyde, silica dust |
| Mutagenic | An agent that can change the genetic material (DNA) in a cell. | Permanent, heritable genetic damage | Ethylene oxide, certain pesticides, some industrial dyes |
| Reprotoxic | A substance that is toxic to reproductive functions and fertility. | Impaired fertility, developmental harm to an unborn child | Lead compounds, certain phthalates (plasticisers), some solvents like toluene |
Each of these categories presents a serious challenge, but together, they form a group of high-consequence chemicals that demand the highest level of control and scrutiny in any workplace.
The Real-World Business Challenge
For any organisation, managing CMRs boils down to two core problems. First, there's the ever-shifting maze of regulations. Rules like REACH and CLP in Europe are constantly being updated. A chemical that was perfectly fine to use last year could be reclassified as a CMR tomorrow, instantly making your products or processes non-compliant. Trying to keep up manually is a recipe for failure.
Second, just finding these substances in your inventory is a monumental task. A CMR might not be the main ingredient; it could be a tiny, undeclared component in a cleaning agent or a residue left over from a manufacturing process. Simply trusting a supplier's Safety Data Sheet isn't enough—they can be outdated, incomplete, or just plain wrong. This creates a massive compliance and safety blind spot.
The fundamental principle driving all CMR legislation is precaution. Because the potential harm is so severe and irreversible, the law puts the burden on the employer to prove a substance can be used safely—not on the worker to prove it caused their illness.
Tackling CMR management head-on isn't just about ticking a compliance box. It’s a critical part of broader ESG strategies for sustainability and responsibility. By truly understanding why these chemicals are so heavily regulated, you can build a more resilient and effective safety culture from the ground up.
How to Reliably Identify CMR Substances in Your Inventory
Finding CMR substances in your chemical inventory can feel a lot like detective work. These high-risk chemicals aren't usually advertised on the front of a container; their presence is often buried deep within technical documents. But with the right strategy, you can turn this overwhelming task into a clear, manageable process and gain the confidence that your team is protected.
The starting point for any investigation is always the Safety Data Sheet (SDS). Think of an SDS as a chemical's biography—it tells you everything you need to know about its identity, hazards, and the rules for handling it safely. The trick is knowing exactly where to look for the CMR clues.
Decoding the Safety Data Sheet
When you pick up an SDS, you don't need to read it cover-to-cover. To find out if you're dealing with a CMR, you can zero in on two key sections.
- Section 2: Hazard Identification: This is your first stop. It contains the official hazard classifications under the Globally Harmonised System (GHS). You're looking for specific hazard statements (H-statements) that act as clear red flags for CMR properties.
- Section 11: Toxicological Information: This section gives you the "why" behind the classifications in Section 2. It provides the scientific evidence and details about the carcinogenic, mutagenic, or reprotoxic effects that have been identified.
The most direct way to confirm a CMR is by spotting one of the specific H-statements assigned to these hazards. These codes are the universal language for chemical risk.
Key CMR Hazard Statements to Watch For:
- H350: May cause cancer (Carcinogenic)
- H340: May cause genetic defects (Mutagenic)
- H360: May damage fertility or the unborn child (Reprotoxic)
If you see any of these codes, you have a confirmed CMR substance. That means you need to treat it with the highest level of caution and follow strict regulatory protocols for its management.
Using CAS Numbers for Verification
While the SDS is your primary tool, it shouldn't be your only one. Supplier information can sometimes be incomplete or out of date, which is a massive compliance risk. This is where the Chemical Abstracts Service (CAS) number becomes so important.
A CAS number is a unique identifier assigned to every distinct chemical substance. You can think of it as a chemical's fingerprint—no two are the same. This allows you to check the substances in your inventory against official regulatory databases, completely bypassing any potential errors on the supplier's SDS. It’s a crucial step for independent verification.
Some of the most reliable databases for this cross-referencing include:
- ECHA C&L Inventory: The European Chemicals Agency's Classification and Labelling Inventory is a massive, publicly accessible database with information on substances registered under regulations like CLP.
- REACH Candidate List: This is the official list of Substances of Very High Concern (SVHCs). A huge number of these are classified as CMRs and are flagged for potential restriction or phase-out.
Let's be honest, manually checking every single substance against these lists is incredibly time-consuming and prone to human error, especially if you manage hundreds of chemicals. This is exactly why many organisations now rely on a modern chemical inventory management system to automate the screening process. It’s a far more robust way to ensure no CMR slips through the cracks.
Don't assume that even official data is always complete. A concerning case in Belgium revealed that 17.9% of notifications for tobacco products were missing crucial studies on toxicity and CMR properties. This just goes to show how challenging it can be for EHS managers to get a clear picture of risk when relying on supplied data alone. It really hammers home the need for diligent, independent verification. You can learn more about these compliance challenges from research on the topic.
Navigating Your Legal and Regulatory Obligations
Knowing a substance is a CMR is one thing; understanding what to do about it is the real challenge. For any business operating in Belgium or across the EU, this means getting to grips with a complex web of legal duties. Frameworks like REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) and CLP (Classification, Labelling and Packaging) aren’t just best-practice guides—they're the law.
These regulations place a very clear responsibility on employers. Your primary legal duty isn't just to manage the risk posed by CMR substances, but to eliminate them from your workplace entirely wherever possible. This is the principle of substitution.
The Core Principle: Substitution
At its heart, the law is simple: if you can replace a hazardous CMR substance with a safer alternative, you must. This isn't a friendly suggestion; it's the mandatory first step in your risk management process. You have to be able to prove you've looked for alternatives and have a solid reason if substitution just isn't technically feasible.
This legal pressure is designed to do more than just manage risk. It’s a powerful driver for innovation, pushing industries towards greener chemistry and inherently safer ways of working. The ultimate goal is to design the hazard out of the process, not just put a lid on it.
Key Employer Responsibilities
Beyond substitution, your legal obligations involve a series of concrete actions. These are non-negotiable and form the foundation of any compliant chemical safety programme.
- Conduct Robust Risk Assessments: You must carry out specific, detailed risk assessments for any process involving a CMR. This isn't a box-ticking exercise; it has to dig into the nature, duration, and potential level of exposure.
- Implement Specialist Employee Training: Anyone who might come into contact with CMRs needs proper training. This must cover the specific health risks, safe handling procedures, correct use of Personal Protective Equipment (PPE), and what to do in an emergency.
- Maintain Detailed Exposure Records: For many CMRs, the law requires you to keep records of employee exposure for up to 40 years after the exposure ends. This is vital for long-term health monitoring and, frankly, for managing long-term liability.
The Gatekeeper Role of Procurement
One of the most effective ways to stay compliant is to stop CMR substances from ever setting foot in your facility. This turns your procurement and supply chain teams into the front-line gatekeepers of chemical safety.
Before any new chemical gets the green light, it needs to be properly vetted. Your procurement team should be working hand-in-hand with your Health, Safety, and Environment (HSE) managers to screen potential new products. An unvetted purchase can easily introduce a restricted or banned substance, creating a serious compliance headache overnight.
This simple workflow shows how to screen substances before they become a problem.
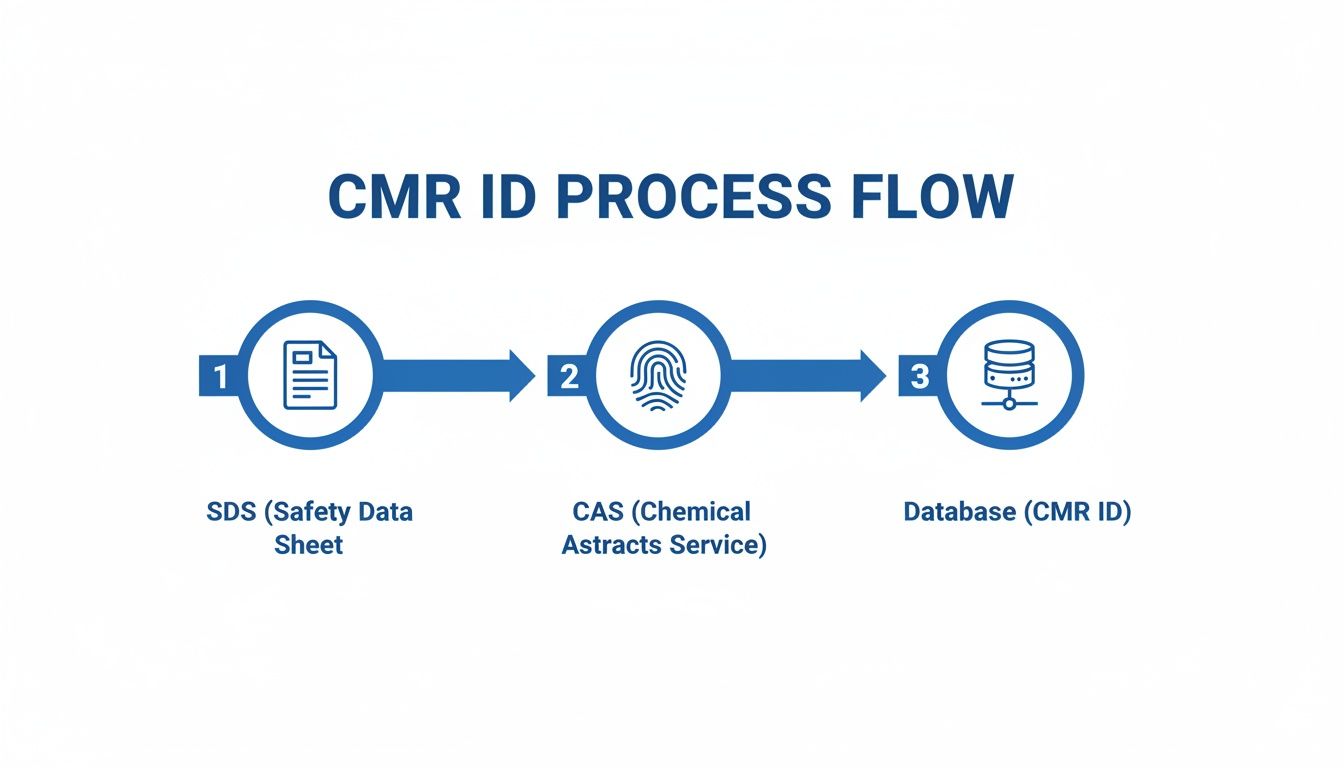
This three-step process—reviewing the Safety Data Sheet, using the CAS number to verify it, and cross-referencing against official databases—is the most reliable way to confirm a substance's status.
The regulatory environment in Europe is anything but static. In Belgium, workplace exposure registries help authorities see which CMRs are most prevalent and focus their risk management efforts. These local initiatives are part of a wider EU push for substitution, highlighted by the 88 new CMR 1A/B substances identified EU-wide between 2009 and January 2023.
Regulations like REACH Annex XVII, which bans certain CMRs in consumer products above specific limits, show just how stringent the rules are. For HSE and procurement teams, navigating this landscape is a daily reality. You can learn more about how EU-wide data informs these regulatory actions and drives the push for safer chemical use across the continent.
Implementing Effective Workplace Control Measures

Once you've identified a CMR substance in your workplace, the real work begins. Knowing the danger is there isn't enough; you have to actively control the risk it poses to your team. The best way to tackle this is with a tried-and-tested framework called the Hierarchy of Controls.
Think of it as a priority list, starting with the most effective, permanent solutions at the top and working down to the least effective. The goal is always to start as high up the list as you can, not just jump to the easiest option at the bottom. This systematic approach is the bedrock of managing the serious risks that come with CMRs.
Starting at the Top: Substitution and Elimination
The gold standard in risk management is elimination. If you can completely remove the hazardous chemical or the process that uses it, the risk vanishes along with it. A perfect example would be a furniture maker who stops using a solvent-based adhesive containing a CMR and switches to using mechanical fasteners instead. The chemical hazard is gone. Period.
When getting rid of the process entirely isn't on the table, the next best thing is substitution. This means swapping out a dangerous CMR substance for a safer, non-CMR alternative that still gets the job done. Think of replacing benzene—a well-known carcinogen used as a solvent—with less toxic options like heptane for certain tasks.
The legal and ethical duty is clear: always try to eliminate or substitute first. These are the only two strategies that get to the root of the problem, offering the most reliable and permanent protection from CMR substances.
Just be thorough. When you substitute one chemical for another, you have to be sure the new one doesn't bring its own set of unexpected dangers. A full risk assessment is crucial to ensure you're making things genuinely safer.
Hierarchy of Controls for CMR Substances
To manage CMRs effectively, it's vital to apply controls in order of preference, from most to least effective. This table breaks down the hierarchy, providing a clear roadmap for your risk management strategy.
| Control Level | Description | Practical Example for CMRs |
|---|---|---|
| Elimination | Physically remove the hazard from the workplace entirely. | Switching from a CMR-containing solvent to a water-based cleaning process. |
| Substitution | Replace the hazardous substance with a less hazardous one. | Replacing a formaldehyde-based resin with a safer, bio-based alternative. |
| Engineering Controls | Isolate people from the hazard by making physical changes to the workplace or equipment. | Installing a closed-loop system for chemical mixing or using local exhaust ventilation (LEV) to capture fumes at the source. |
| Administrative Controls | Change the way people work, such as through procedures, training, or job scheduling. | Limiting access to high-risk areas, implementing strict safe-handling procedures, or rotating jobs to reduce exposure time. |
| Personal Protective Equipment (PPE) | Protect the worker with equipment like gloves, goggles, and respirators. | Providing chemical-resistant gloves and a properly fitted respirator for handling a carcinogenic liquid. |
Always remember that relying on measures at the bottom of the hierarchy, like PPE, indicates that more significant risks are still present in the workplace.
Engineering Controls: Isolating the Hazard
If you can't eliminate or substitute the CMR, your next line of defence is engineering controls. These are physical changes to your workspace, equipment, or processes that are designed to put a barrier between your employees and the hazard. The beauty of these controls is that they don't depend on someone remembering to do something—they're built right into the environment.
Common engineering controls for CMRs include:
- Closed-Loop Systems: Imagine a system where the CMR chemical is contained from start to finish, transferred and mixed within sealed pipes and vessels. This prevents any dust or vapour from ever reaching the open air.
- Local Exhaust Ventilation (LEV): This isn't just a general fan. LEV systems are designed to capture contaminants right where they are produced—think of a fume hood in a lab or an extraction arm positioned over a mixing tank. It sucks the hazardous air away before anyone can breathe it in.
These solutions are incredibly effective because they engineer the risk out of the day-to-day process, offering a constant and reliable form of protection.
Administrative Controls and Safe Work Practices
Further down the hierarchy, we find administrative controls. These are all about changing how people work through procedures, policies, and training. While not as robust as engineered solutions, they are a critical layer in any good safety programme.
Examples include things like:
- Restricted Access Zones: Clearly marking off areas where CMRs are handled and ensuring only trained, authorised staff can enter.
- Safe Work Procedures (SWPs): Writing down clear, step-by-step instructions for any task involving a CMR, from decanting it safely to cleaning up a spill correctly.
- Job Rotation: Making sure no single person spends too much time on a task with high exposure potential.
These controls rely heavily on people following the rules, which makes them vulnerable to human error. For a deeper dive into creating these systems, check out our guide on improving chemical safety in the workplace.
Personal Protective Equipment: The Last Defence
Right at the bottom of the pyramid is Personal Protective Equipment (PPE). We’re talking about respirators, chemical-resistant gloves, aprons, and bodysuits. PPE is absolutely essential, but it should only ever be your last resort.
Why? Because relying on PPE alone is a high-stakes gamble. Its effectiveness hinges on everything going right: choosing the exact right type of gear, making sure it fits perfectly, training employees to use it every single time, and maintaining it properly. If any one of those steps fails, the protection is gone. The specific PPE you need will be listed in Section 8 of the chemical’s Safety Data Sheet (SDS), and you must follow that guidance to the letter.
Getting Labelling, Storage, and Disposal Right
Identifying a CMR substance is only the first step. The real work begins when you have to manage it throughout its entire lifecycle in your facility. This means getting the practical details of labelling, storage, and disposal absolutely correct. Think of it as a chain of responsibility – every link has to be strong to keep people safe.

From the moment a CMR chemical arrives on-site, it needs to be clearly and correctly labelled. This isn't just good practice; it's a legal obligation under regulations like CLP (Classification, Labelling and Packaging). The aim is to give anyone who comes near it an immediate, impossible-to-misunderstand warning about the serious, long-term health risks involved.
The Language of a Label
A proper label is your first and most important line of defence. For any container holding a CMR, the label must be crystal clear and follow strict rules.
- The Health Hazard Pictogram: You'll immediately recognise the GHS08 pictogram: the silhouette of a person with a starburst on their chest. This is a non-negotiable, universal symbol that screams "long-term health hazard," specifically covering carcinogens, mutagens, and reproductive toxicants.
- Signal Words & Hazard Statements: The label also needs a signal word, which is usually "Danger" for the most serious CMRs (Categories 1A and 1B). This is paired with specific hazard statements like H350 (May cause cancer). These phrases provide the critical context that the picture alone can't convey.
- Secondary Containers: Don't forget this applies to any container. If you transfer a CMR from a large drum into a smaller spray bottle for use on the factory floor, that smaller bottle must carry a label with all the same essential hazard information. No exceptions.
Clear, compliant labelling isn't just about ticking a box. It ensures that everyone, from a veteran chemical engineer to a first responder in an emergency, instantly grasps the severity of the hazard they’re dealing with. An unlabelled bottle is a ticking time bomb and a massive compliance failure.
Secure and Segregated Storage
Once they're labelled, you can't just stick CMR substances on any old shelf. Storing them requires a deliberate, controlled strategy built around one core principle: containment and segregation.
Proper storage is all about creating robust physical barriers between these hazardous materials and your people.
- Designated Areas: CMRs belong in designated, clearly marked areas with restricted access. These should be locked or otherwise secured to keep unauthorised staff out.
- Segregation from Incompatibles: This is crucial. You must store CMRs away from chemicals they could react dangerously with. For instance, putting a flammable carcinogen next to a powerful oxidiser is asking for a fire. Always check Section 7 of the Safety Data Sheet for specific advice on what to keep separate.
- Ventilation and Containment: The storage area needs good ventilation to stop hazardous vapours from building up. It also needs secondary containment, like spill pallets or a bunded floor, to catch any leaks and prevent a small problem from becoming a major incident.
Compliant Disposal Protocols
The final stage, disposal, is every bit as tightly regulated as handling and storage. You can’t just wash CMR waste down the sink or toss it in the general waste skip. These materials are legally classified as hazardous waste.
The only safe and legal route is to work with a licensed, certified waste management contractor. These specialists have the right permits, equipment, and knowledge to handle, transport, and dispose of CMR substances correctly. They will provide you with all the necessary paperwork, like waste transfer notes, which you must keep on file. This creates a clear, auditable paper trail, proving you've managed your responsibilities properly from start to finish.
How Digital Tools Streamline CMR Compliance
Trying to manage CMR substances with spreadsheets and manual checks is a bit like navigating a motorway using a paper map from the 90s. It’s not just clunky and inefficient; it’s a genuinely high-risk strategy when the rules of the road are changing all the time. Thankfully, modern technology can transform this headache into a structured and largely automated process.
With the right digital platform, you stop playing catch-up and start getting ahead of compliance. These systems act as a single source of truth for your entire chemical inventory, putting an end to the frantic search for outdated Safety Data Sheets (SDSs) that are inevitably scattered across different sites or buried in a filing cabinet.
Automating the Screening Process
If there’s one thing that keeps health and safety managers up at night, it’s the constant churn of regulations. A substance that’s perfectly fine to use today could land on the REACH Candidate List or get a new CLP classification tomorrow. Keeping track of this manually across hundreds or thousands of products is a task that’s doomed to fail.
This is where digital tools really earn their keep. They automate the heavy lifting by continuously cross-referencing your chemical inventory against hundreds of regulatory lists from around the world.
- Real-Time Monitoring: Forget about once-a-year audits. A good system is always on, checking for updates to key lists like REACH, SVHC, and CLP every single day.
- Instantaneous Alerts: The moment a chemical in your stock is reclassified as a CMR, your HSE team gets an alert. This means you won't get caught out, still using a newly restricted substance weeks or months later.
- Supplier Validation: These tools can also spot when a supplier’s SDS is out of date or, worse, doesn't match the official regulatory classification for a substance. It ensures the data you rely on is actually correct.
This constant, automated vigilance closes the dangerous time lag between a new regulation being published and your company actually doing something about it.
Imagine a system that uses Artificial Intelligence to literally read and understand any SDS you upload. It doesn't just save the PDF; it intelligently pulls out the crucial data—CAS numbers, hazard statements, exposure limits—and populates your chemical database in seconds. That's what modern compliance tech can do.
To get a better feel for how these platforms operate, it’s worth looking into the features of modern SDS management software, which really is the foundation of digital chemical safety.
Bridging the Gap Between Policy and Practice
Knowing the regulations is one thing, but making sure they're followed on the ground, every day, is the real challenge. Digital tools are the bridge that connects your high-level safety policies to the real-world actions of your team.
Here’s a practical example. A procurement manager is about to order a new cleaning agent. Before hitting 'confirm', they upload the supplier's SDS into the system. Within seconds, the platform scans the document, identifies an ingredient classified as a Category 1B CMR, and flags it as a restricted substance. The purchase is stopped in its tracks, preventing a non-compliant chemical from ever setting foot in your facility.
This kind of proactive gatekeeping is simply impossible with a paper-based system. For a deeper dive into how this works for hazardous materials, there's more information on AI Security and Compliance in the Chemical Pharma Process Industry. By building these checks directly into your workflows, you make safety the default setting, not an afterthought.
Your Top Questions About CMRs Answered
Even with a good handle on the basics, the day-to-day management of CMR substances can throw up some tricky questions. Getting the details right is what separates a truly effective safety programme from one that just ticks the boxes.
Let’s tackle some of the most common queries that come up in the field. Think of this as a practical guide to help you navigate those specific scenarios with confidence.
What’s the Difference Between CMR Categories 1A, 1B, and 2?
Think of these categories as a measure of certainty—how strong is the scientific evidence linking a substance to harm?
Category 1A (Proven Hazard): This is the highest level of certainty. We have solid evidence from studies on people that directly links the substance to causing cancer, genetic mutations, or reproductive damage. There’s no ambiguity here.
Category 1B (Presumed Hazard): While we might not have direct proof from human studies, the evidence from animal testing is so strong that we have to presume it will have the same effect on humans.
Category 2 (Suspected Hazard): Here, the evidence is more limited. There are some worrying signs from human or animal studies, but it’s not conclusive enough to push it into Category 1. It’s a case of "watch this space."
Unsurprisingly, regulations hit Categories 1A and 1B the hardest. Under rules like REACH, these substances are often treated as one and the same, facing outright bans or extremely tight restrictions on their use.
How Often Should We Check Our Inventory for CMRs?
The simple answer? All the time. Relying on an annual review is a dangerously outdated approach. Regulatory lists are living documents; authorities like ECHA update them constantly. A supplier could also send you a revised Safety Data Sheet tomorrow, reclassifying a product you use every day.
The best practice is to have an automated system in place that monitors your inventory in real-time. You need alerts that tell you the moment a chemical you hold has been reclassified. If you're still stuck doing this manually, a full inventory audit is the bare minimum annually, but you also need to do it every single time a new chemical comes through the door.
Is There a Safe Exposure Level for a CMR Substance?
For most carcinogens and mutagens, the scientific and regulatory agreement is clear: there is no safe level of exposure. This isn't like other toxins where a small amount might be harmless. With many CMRs, the theory is that even a single molecule could be enough to start a chain reaction that leads to disease.
This is where the legal principle of ALARP—"as low as reasonably practicable"—comes in. Your first duty is always to get rid of the substance entirely or swap it for a safer alternative. If that's impossible, your goal shifts to using engineering controls and strict procedures to reduce any potential contact to the absolute minimum.
What if a Supplier's SDS Doesn't Match a Regulatory List?
When there’s a conflict, the official regulatory list is king. Always. A supplier's Safety Data Sheet can be out of date, have errors, or just be plain wrong. Your legal responsibility is to follow the official classification published by the governing bodies.
If you spot a mismatch, you need to act immediately. Update your risk assessment using the official data, put the right control measures in place for that higher level of risk, and then get on the phone with your supplier to demand a corrected and compliant SDS.
Navigating these complexities is exactly why we built NextSDS. Our platform takes the guesswork and manual slog out of compliance by automatically screening your inventory against global regulatory lists in real-time. You'll never be caught out by a classification change again. Stop wondering if you're compliant—know for sure. Visit https://nextsds.com to see how we make chemical safety straightforward.

