The world of EU chemical regulations can seem like a labyrinth, but it boils down to two key concepts: REACH and SVHC. Think of the REACH Directive as the master blueprint for chemical safety in Europe. It's a massive, comprehensive regulation that covers basically all chemical substances. Then, within that blueprint, you have the SVHC list—a much more focused list that flags the "most wanted" chemicals with serious health or environmental risks.
When a substance lands on the SVHC list, it sets off a chain reaction of legal duties for companies. If your product contains one of these substances above a 0.1% concentration, you're on the hook to notify your customers and manage the associated risks. The burden of proof is squarely on businesses to know what's in their products and to communicate that information down the supply chain.
What Are the REACH Directive and SVHC List?
Let's break this down. At the top level, you have REACH, which stands for Registration, Evaluation, Authorisation and Restriction of Chemicals. It’s the EU’s framework designed to protect people and the environment from chemical hazards. The core idea is to make companies responsible for understanding and managing the risks of the chemicals they manufacture and sell.
Now, nested inside REACH is the SVHC Candidate List. SVHC means Substances of Very High Concern. These are the chemicals that raise the biggest red flags because they are known to be:
- Carcinogenic (cause cancer)
- Mutagenic (cause genetic mutations)
- Toxic for reproduction (harm fertility or unborn children)
- Persistent, Bioaccumulative and Toxic (PBT), meaning they stick around in the environment and build up in wildlife and people.
To get a clearer picture of the relationship between these two, let's compare them side-by-side.
REACH vs SVHC at a Glance
This table helps illustrate how the broad REACH regulation and the specific SVHC list fit together.
| Aspect | REACH Directive | SVHC Candidate List |
|---|---|---|
| Scope | A broad EU regulation covering all chemical substances manufactured or imported into the EU. | A specific, targeted list of substances identified as having hazardous properties. |
| Purpose | To improve the protection of human health and the environment through better risk management. | To identify substances for potential inclusion in the Authorisation List (Annex XIV), which may lead to future restrictions. |
| Immediate Obligation | Registration of substances above one tonne per year and communication of risks. | Communication down the supply chain and consumer notification if present in articles above 0.1% w/w. |
Essentially, REACH is the entire legal framework, while the SVHC list is a critical, high-priority component within it that triggers very specific actions.
The Watchlist Analogy
A great way to think about the SVHC list is as a regulatory watchlist. Getting on this list doesn't automatically mean a chemical is banned. Instead, it’s a formal declaration that the substance is under intense scrutiny. It immediately triggers legal obligations for any company whose products contain it above that crucial 0.1% weight-by-weight (w/w) threshold.
Being on the "watchlist" is the first step toward potentially tighter controls, such as requiring special authorisation for use or even a future ban.
The European Chemicals Agency (ECHA) is the gatekeeper of this list, and they update it twice a year. This is a huge deal for businesses—a chemical that was perfectly fine to use yesterday could suddenly bring a whole new set of compliance headaches tomorrow. This is exactly why staying on top of the list is not just good practice, it's a business necessity.
Here’s a snapshot of what the official Candidate List looks like on ECHA's website.
As you can see, the table lays everything out clearly: the substance name, its identifiers, why it was included, and the date it was added. For compliance teams, this is the source of truth.
From Data Sheets to Compliance Checks
So, where do you even start? How does a company figure out if its products contain an SVHC? It all begins with good supplier communication and having the right paperwork, especially the Safety Data Sheet (SDS).
But just knowing what is a Safety Data Sheet is only the first step. The real work—and the real headache—is in systematically digging through the ingredient data from potentially hundreds of these documents. You have to extract the right information and constantly check it against the ever-changing SVHC list. This is where procurement managers and safety teams often discover the true scale of the REACH challenge.
How a Substance Gets Added to the SVHC List
The SVHC list isn't static. It's a living document that gets updated as our scientific understanding of chemical risks evolves. The European Chemicals Agency (ECHA) is in charge of a rigorous, multi-stage process for flagging and listing new substances, making sure every addition is backed by solid evidence. For any business, understanding how a chemical makes this journey is key to staying ahead of compliance.
Think of it like a funnel. Out of the thousands of chemicals used across the EU, only a small fraction that meets specific, serious hazard criteria even gets considered for the SVHC list. Getting a handle on this filtering process is what allows savvy companies to do some "horizon scanning" and predict which materials in their supply chain might be next in line for regulation.
This diagram shows the big picture—how the vast universe of chemicals is narrowed down through the REACH regulation to pinpoint the most hazardous ones for the SVHC list.
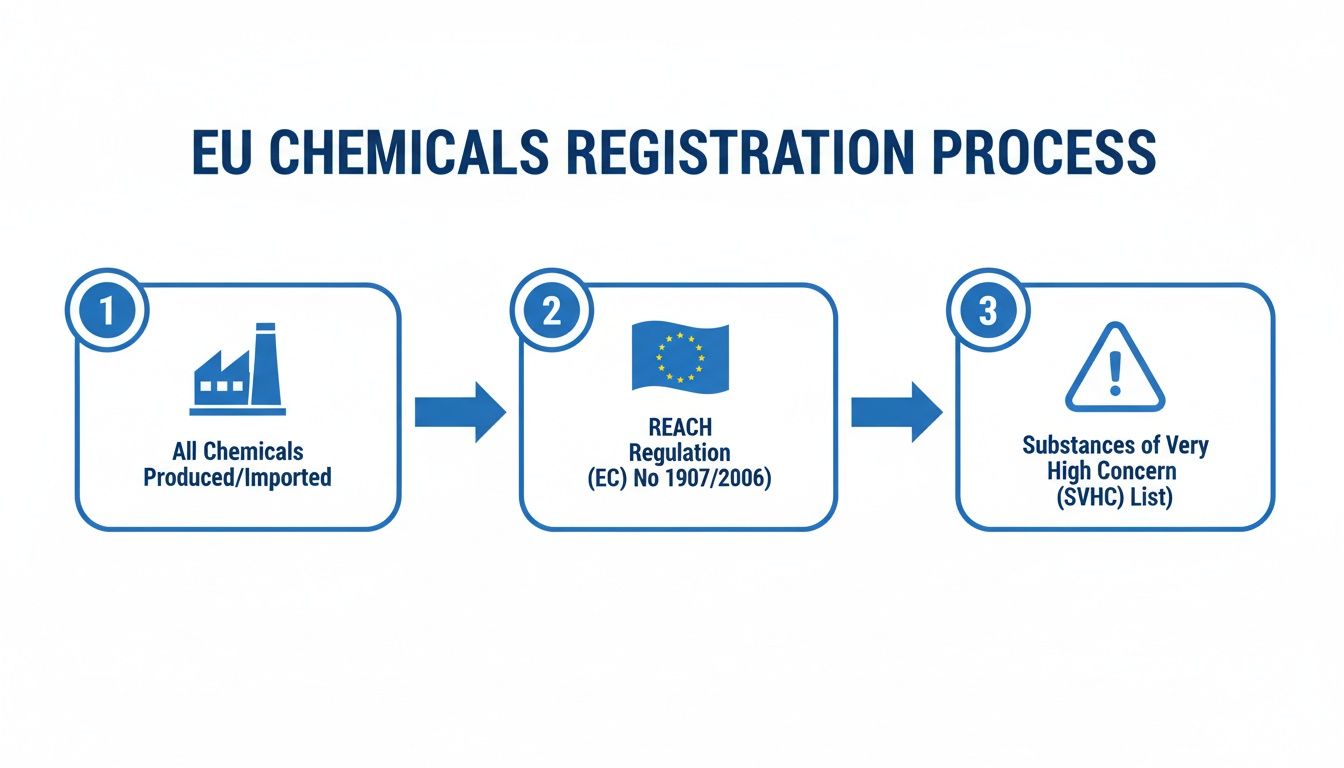
As you can see, it’s a deliberate process designed to focus regulatory attention where it’s needed most. Once a substance lands on this list, it triggers a whole new set of legal duties for companies up and down the supply chain.
The Four Stages of SVHC Identification
Getting a substance onto the SVHC list is a methodical and transparent affair. It's not a snap decision; it's a careful evaluation with several key steps that allow for scientific debate and industry feedback.
A Proposal is Made: It all starts when an EU Member State, or ECHA itself, puts together a detailed dossier. This document lays out the scientific case for why a substance meets the tough criteria defined in Article 57 of the REACH regulation.
Public Consultation Kicks Off: This is where transparency comes in. ECHA publishes the proposal and opens the floor to anyone with a stake in the outcome—companies, industry groups, scientists, and the public. For 45 days, anyone can submit comments or new data.
The Member State Committee (MSC) Weighs In: After the consultation closes, the MSC, made up of experts from every EU country, gets to work. They review the proposal and all the feedback, aiming to reach a unanimous decision on whether the substance is truly an SVHC.
It’s Added to the Candidate List: If the MSC agrees, the substance is officially added to the SVHC Candidate List. Simple as that. If they can’t find consensus, the decision gets passed up to the European Commission to make the final call.
This step-by-step approach ensures that every decision is grounded in evidence and that everyone has a chance to be heard before new compliance headaches are created.
The Criteria for Becoming an SVHC
So, what exactly lands a substance on the radar in the first place? The criteria are very specific and target severe, often irreversible, dangers to our health and the environment.
A substance can be proposed if it falls into one of these categories:
- Carcinogenic, Mutagenic, or Toxic to Reproduction (CMR): These are the big ones—chemicals known to cause cancer, trigger genetic mutations, or harm fertility and unborn children.
- Persistent, Bioaccumulative, and Toxic (PBT): This category is for substances that stick around in the environment for a long time, build up in the food chain, and are toxic.
- Very Persistent and Very Bioaccumulative (vPvB): A similar class of chemicals that are extremely stubborn in the environment and accumulate significantly in living organisms.
- Equivalent Level of Concern: This is the "catch-all" for other substances that pose a similar level of risk but don't fit neatly into the other boxes. Think endocrine disruptors or potent respiratory sensitizers.
This rigorous process for identifying SVHCs under the REACH directive typically happens twice a year, with updates usually landing in January and June. For example, ECHA’s 34th update on November 5, 2025, added DBDPE, bringing the list to 251 SVHC entries—though some of these are group entries covering multiple chemicals. You can learn more about the REACH SVHC listing process and timelines.
Understanding Your Core Compliance Obligations
The moment a substance in your supply chain gets added to the SVHC list, the clock starts ticking. This isn't something you can pencil in for next quarter; a new set of legal duties kicks in immediately. Everything hinges on one critical number: the 0.1% weight by weight (w/w) concentration threshold in your products, or what the regulation calls "articles."
If an SVHC is present above this tiny limit, you can't just carry on with business as usual. The REACH directive demands that you proactively communicate up and down the supply chain, ensuring everyone from your industrial clients all the way to the end consumer understands the potential risks. Getting a firm grip on these duties is the first, most crucial step in building a compliance strategy that actually works.

The Three Pillars of SVHC Compliance
Your responsibilities really boil down to three main areas. Each one is designed to create a web of transparency and safety that follows a product from the factory floor to its final disposal. It helps to think of them as interconnected tasks that form a complete safety net.
- Supply Chain Communication (Article 33): This is your most pressing job. You're required to give your downstream users—the companies buying your products—enough information to use them safely. At the very least, this means telling them the name of the SVHC.
- ECHA Notification (Article 7): This one is for producers or importers of articles containing an SVHC. If the total amount of that specific SVHC across all your articles adds up to more than one tonne per year, you have to formally notify the European Chemicals Agency (ECHA).
- SCIP Database Submission: The SCIP database is the EU’s central library for information on articles that contain SVHCs. Suppliers have to upload detailed information here, which helps promote a circular economy and gives waste operators a heads-up on handling hazardous materials.
These pillars are the absolute foundation of your compliance program. Dropping the ball on any one of them can lead to some hefty penalties and even get your products blocked from the EU market. For a broader look at the types of rules businesses face, you can find helpful overviews on legal and regulatory compliance.
Decoding the 0.1% Threshold in Practice
That 0.1% rule is a frequent source of headaches. It’s not calculated based on the total weight of your final product. Instead, it applies to each individual "article" that makes up the finished product. An article is defined as an object whose special shape, surface, or design is more important to its function than its chemical makeup.
Let’s take a power drill as an example. The plastic casing is one article. The power cord is another. A tiny capacitor on the circuit board? That’s an article, too. If the rubber on that power cord contains an SVHC at 0.2% of the cord's weight, you have a duty to communicate that—even if that amount is practically nothing compared to the weight of the entire drill.
The REACH Candidate List is always growing, now standing at 251 entries. If any single article in your product contains an SVHC above the 0.1% w/w threshold, suppliers are obligated under Article 33 to inform their customers right away. On top of that, if you import or produce over 1 tonne per year of that SVHC, you have six months from its listing to notify ECHA.
This incredibly detailed requirement means you absolutely must have comprehensive data on your products. You need a complete Bill of Materials (BOM) and detailed substance declarations from every single supplier to have any chance of assessing your obligations accurately. This same level of detail is also vital for correctly filling out your Safety Data Sheets; knowing what goes into Section 15 regulatory information is a huge part of being fully compliant.
For anyone making complex products, the challenge gets bigger fast. A single car contains thousands of articles—from the fabric on the seats and the plastic on the dashboard to the gaskets in the engine. Each one has to be checked against the SVHC list. This is where manual tracking breaks down and compliance becomes a massive data management nightmare, highlighting why automated screening tools are no longer a luxury but a necessity to keep your business safe.
Moving Beyond Spreadsheets for SVHC Compliance
Let's be honest: trying to manage REACH directive SVHC compliance with spreadsheets and manual supplier checks is a recipe for disaster. With the SVHC list growing twice a year, that old-school approach is more than just slow—it’s a genuine risk to your market access and your company's reputation. You end up stuck in a constant cycle of putting out fires instead of getting ahead of the problem.

This manual process is full of holes. When you're manually cross-referencing hundreds of chemicals against an ever-expanding list, human error is practically a given. Worse, information gets stuck in silos. Procurement might have an updated Safety Data Sheet (SDS) that the HSE team hasn't seen, creating massive blind spots in your safety and compliance picture. Simply keeping up becomes a full-time, resource-draining job.
Making the Switch to Automated Screening
Adopting a modern, automated system isn't just an upgrade; it's a fundamental shift in how you operate. Instead of having someone manually hunt through documents, compliance platforms can ingest your entire library of Safety Data Sheets. These systems use smart technology to pull out the crucial details—like CAS numbers and chemical ingredients—and organize everything into a clean, searchable database.
From there, the platform continuously cross-references your chemical inventory against the latest SVHC list and other global regulations. It turns compliance from a stressful, twice-a-year scramble into a quiet, background process that’s always on.
The real power of automation lies in moving from reactive damage control to proactive risk management. You stop finding out about an SVHC in your product after it's already on the shelf. Instead, you get an immediate alert the moment an SDS listing a new SVHC hits your system.
This instant awareness gives your teams the information they need, right when they need it:
- HSE Managers get immediate flags on new risks, so they can update safety protocols and risk assessments without delay.
- Procurement Teams can screen materials before they even place an order, stopping non-compliant chemicals at the door.
- Logistics Teams have accurate, current data ready for safe handling, storage, and transport paperwork.
From Manual Drudgery to a Strategic Edge
Automated platforms offer more than just a simple checklist. They create a single, reliable source of truth that everyone in the organization can trust. In the world of SVHC compliance, finding ways to improve workflow efficiency is non-negotiable, and this is how you do it. Your skilled professionals are freed from mind-numbing data entry and can finally focus on what they do best: strategic planning and finding safer chemical alternatives.
The table below breaks down just how stark the difference is between the old way and the new way.
Manual vs Automated SVHC Compliance
| Compliance Task | Manual Method (Spreadsheets) | Automated Platform (NextSDS) |
|---|---|---|
| New SDS Onboarding | Someone has to read every SDS, identify chemicals, and manually check them against the SVHC list. | The system automatically scans the SDS, extracts all chemical data, and flags any SVHCs instantly. |
| List Updates (Bi-annual) | A team member must find the new list, then re-check every product in the inventory. This can take weeks. | The platform’s database is updated automatically. Your entire inventory is re-screened in minutes, with alerts for new matches. |
| Supplier Communication | A long back-and-forth email chain to confirm if a product contains a newly added SVHC. | You already have the data. You can proactively ask suppliers about alternatives instead of just asking for confirmation. |
| Reporting & Audits | A stressful scramble to pull data from multiple spreadsheets, hoping nothing was missed. | Generate a comprehensive compliance report with a few clicks, with full traceability for every product. |
| Human Error Risk | High. Typographical errors, missed chemicals, and outdated lists are common and costly. | Extremely low. The process is standardized and automated, removing the potential for manual mistakes. |
As you can see, automation builds a scalable system that adapts as your business—and the regulations—evolve. When ECHA adds more substances to the REACH directive SVHC list, your system just handles it. No more frantic searches or last-minute panics. That kind of control is essential for any business serious about competing in the EU market.
This automated workflow is a key piece of a bigger puzzle. It works hand-in-hand with a well-organized digital inventory, a cornerstone of any good chemical inventory management software. Ultimately, this shift turns compliance from a costly operational headache into a proactive, strategic advantage that protects both your revenue and your reputation.
What Happens After a Substance is Labeled an SVHC?
Making it onto the SVHC Candidate List is a big deal, but it's really just the beginning of the regulatory journey. Think of the Candidate List as the official "watch list." As soon as a substance is on it, new communication duties kick in. But for smart, long-term planning, you need to look ahead at what comes next.
Two critical paths lead from the Candidate List: the Authorisation List (Annex XIV) and the Restriction List (Annex XVII). Knowing the difference between them is absolutely crucial for future-proofing your products and your supply chain. If you aren't watching for a substance's potential move to one of these lists, you could face sudden, disruptive bans on materials central to your business.
The Authorisation List: Getting a Special Permit to Operate
When a substance moves from the Candidate List to the Authorisation List, the game completely changes. This list, formally called Annex XIV of REACH, effectively bans the substance from being placed on the EU market after a specified "sunset date."
It’s like a special permit system. Once that sunset date passes, you can't use the substance anymore—unless you’ve gone through the process of applying for and receiving a specific authorisation from ECHA. This is not a simple paperwork exercise; it's a complex, expensive, and time-consuming ordeal. You have to prove that:
- The risks from using the substance are properly controlled.
- There are no technically or economically feasible alternatives.
Getting an authorisation is a high hurdle, and there’s no guarantee of success. The whole point of this process is to systematically phase out the most hazardous chemicals, forcing industries to find and switch to safer alternatives.
The moment a substance you rely on moves to the Authorisation List, a countdown timer starts. That sunset date is a hard deadline to either secure an elusive permit or find a way to reformulate your products. It’s the ultimate regulatory push toward proactive material replacement.
The Restriction List: A Straightforward Ban
While authorisation is a conditional phase-out, a restriction is often much more direct. The Restriction List, or Annex XVII, works more like a set of specific prohibitions. It can impose a complete ban on a chemical or set strict conditions on its manufacture, use, or sale.
Restrictions are designed to address the specific risks of a substance. For instance, a restriction might:
- Ban a certain phthalate from being used in children's toys.
- Limit the concentration of nickel allowed in jewelry that touches the skin.
- Forbid specific azo dyes in textiles and clothing.
Unlike the Authorisation List, which is exclusively for SVHCs, restrictions can be applied to any substance that poses an unacceptable risk to people or the environment. This makes Annex XVII a powerful and adaptable tool for regulators to manage chemical hazards as they are identified.
For any procurement or HSE team, both of these lists represent the end of the line for many high-risk chemicals. A substance’s journey from the SVHC Candidate List to either Authorisation or Restriction is a clear signal. Regulators are telling the market that the chemical's time is up, making the hunt for safer alternatives not just a best practice, but a core survival strategy.
How Past Deadlines Shaped Modern Compliance
The complex world of REACH directive SVHC compliance we navigate today wasn't built in a day. It’s the direct result of a series of phased registration deadlines that completely changed the game for the European chemical industry. These deadlines were the first real test of the REACH framework, forcing thousands of companies to get intimately familiar with their chemical data in ways they never had before.
This massive, systematic data-gathering exercise was the engine that kickstarted the entire REACH system. For the first time, manufacturers and importers had a legal mandate to collect detailed safety and usage information for their chemicals and report it all to the European Chemicals Agency (ECHA). It was a huge undertaking, but it created the enormous, centralized data repository we have today.
That very repository is now the primary well from which ECHA draws when identifying potential Substances of Very High Concern. In effect, the deadlines created the data needed to regulate the industry.
The Phased Registration Timeline
The registration process, which has been the heart of REACH since 2007, was cleverly rolled out in three phases. The whole thing was based on how much of a substance a company made or imported, a tonnage-based approach that put the highest-volume chemicals under the microscope first.
Here’s how it broke down:
- Phase 1 (December 1, 2010): This first wave targeted the big fish—substances produced or imported in quantities over 1,000 tonnes per year. This naturally captured the largest industrial players and the most common chemicals.
- Phase 2 (May 31, 2013): The net widened to include substances in the 100-1,000 tonnes per year range. This brought many medium-sized companies into the fold and significantly expanded the database of registered chemicals.
- Phase 3 (May 31, 2018): This was the final, and by far the largest, phase. It covered substances handled in volumes between 1-100 tonnes per year, impacting a massive number of smaller companies and specialty chemicals.
This tiered approach was a practical way to manage a monumental logistical challenge. Over a decade, the process gathered data on more than 20,000 existing substances. By the final 2018 deadline, ECHA had received over 23,000 registrations, creating a chemical knowledge base of unprecedented scale. You can get more details on how these REACH deadlines reshaped global compliance on ecomundo.eu.
The Lasting Impact on Today's Compliance
The legacy of these deadlines goes far beyond that initial data collection. They set the precedents that define how companies have to operate now. Most importantly, they cemented the Safety Data Sheet (SDS) as the definitive document for passing chemical hazard information up and down the supply chain.
The intense data requirements of the registration deadlines forced companies to get their house in order. That historical pressure is a big reason why robust, data-driven compliance platforms are so critical for managing today's ever-changing SVHC list.
Think of it this way: the headaches of 2010, 2013, and 2018 are directly connected to the tasks on the desks of HSE and procurement teams today. Those early struggles created the data landscape we all work in now and taught everyone a painful lesson: trying to track this stuff with spreadsheets just doesn't work. The lessons learned back then are precisely why modern, automated systems are no longer a luxury but a necessity for managing the dynamic nature of the REACH directive SVHC list.
Frequently Asked Questions About REACH SVHC Compliance
When you're in the trenches dealing with the REACH directive SVHC list, a lot of practical questions pop up. Let's tackle some of the most common challenges that compliance managers, procurement teams, and safety professionals run into day-to-day. Think of this as your quick-reference guide for making the right call.
What Is the Difference Between the Candidate List and the Authorisation List?
It helps to think of the Candidate List as ECHA's official "watch list." The moment a substance lands here, it's a signal to industry. If that substance is in any of your products above the 0.1% threshold, your communication duties kick in immediately. It’s the first clear warning that a chemical is under the microscope and could face much tighter controls down the road.
The Authorisation List (also known as Annex XIV) is the next level up—and it's a big one. Only a handful of substances from the Candidate List get moved here. Once a substance is on the Authorisation List, its use is basically banned after a specific "sunset date," unless your company gets special permission from ECHA through a long and costly application process.
So, the Candidate List is the warning shot. The Authorisation List is where the phase-out begins.
Does the 0.1% Threshold Apply to the Whole Product or Each Component?
This is a crucial point and a common source of confusion. The 0.1% weight by weight (w/w) threshold applies to each individual "article" that makes up a finished product, not the total weight of the product itself. An article is just a component where its shape and design are more important for its function than its chemical makeup.
Let’s use a power drill as an example:
- The plastic housing is one article.
- The electrical cord is a separate article.
- Even a tiny capacitor on the circuit board counts as its own article.
If the rubber insulation on the cord contains an SVHC at 0.2% of the cord's weight, you have a compliance obligation for that cord. It doesn't matter that the amount is almost nothing compared to the weight of the entire drill. This is exactly why you need detailed, component-level data from your suppliers.
How Do I Check If a Chemical Is on the SVHC List?
The definitive source is always the ECHA website, which updates the official SVHC Candidate List twice a year. But let's be realistic: manually cross-checking every single chemical in your inventory against that list is a recipe for headaches and mistakes, especially if you manage a large portfolio of materials.
The most reliable way forward is to use a modern, automated compliance system. By uploading your Safety Data Sheets (SDS), these platforms can screen all your chemical ingredients against the live SVHC list and other global regulations in an instant. You get a clear, accurate picture of your compliance status without the manual grind.
Moving to an automated system changes the game. Compliance stops being a reactive, twice-a-year scramble and becomes a continuous, proactive process. You get alerted to new risks the moment they appear, helping you avoid costly oversights and keeping your business on the right side of the law.
Ready to ditch the manual spreadsheets and endless supplier emails for a smarter compliance workflow? NextSDS offers an all-in-one platform that automates SDS management and continuously monitors your chemical inventory against the latest REACH SVHC list and other global regulations. See how you can achieve proactive compliance and protect your market access by visiting https://nextsds.com.

