Safety data sheet compliance is all about managing hazardous chemical information to meet legal standards. It’s the bedrock of protecting your team and keeping your business out of hot water with regulators. At its core, it means every hazardous substance on your site has an accurate, current, and easily accessible Safety Data Sheet (SDS). For most companies, this is the point where they realize spreadsheets and manual tracking just won't cut it anymore.
Navigating The Real Risks Of SDS Non-Compliance
Let's be blunt: managing Safety Data Sheets can feel like a thankless, never-ending chore. If you're an HSE manager or on the procurement team, you know the pressure to get compliance right is intense. Sticking with outdated spreadsheets or a binder-based system isn't just inefficient—it’s a massive business risk waiting to blow up.
The fallout from getting it wrong is serious and hits from multiple angles. We're talking about everything from eye-watering regulatory fines that drain your budget to operational shutdowns that bring business to a screeching halt. This is far more than a box-ticking exercise. Solid safety data sheet compliance is a fundamental strategy for protecting your people, your supply chain, and your company’s future.
The Financial Stakes Of Non-Compliance
Regulators don't mess around when it comes to hazard communication violations. In 2024, the Occupational Safety and Health Administration (OSHA) ramped up its enforcement, conducting a whopping 34,696 inspections across U.S. workplaces. Those inspections led to $131.4 million in fines, highlighting just how costly a lapse can be for manufacturers and chemical handlers. Maximum penalties can reach $16,550 for a single serious violation and jump to an astonishing $165,514 for willful or repeat offenses. You can dig deeper into these compliance statistics over at Spacelift.io.
A single outdated SDS or a missing document for a new chemical can unravel everything during an audit, leading to penalties that dwarf the cost of a proper compliance system.
And the direct fines are just the beginning. The indirect costs can be even more devastating. Think about these all-too-common situations:
- Production Shutdowns: An inspector walks in, finds a non-compliant label or can't find the right SDS, and shuts down your line until it’s fixed. Every minute of downtime costs money.
- Supply Chain Delays: A critical shipment gets stuck at the border because its SDS doesn't meet the specific format required by the destination country, like REACH or CLP regulations in Europe.
- Workplace Accidents: An employee gets hurt handling a chemical because the proper instructions weren't available. Now you’re facing workers' compensation claims, lost productivity, and potential legal battles.
These aren't hypothetical problems; they happen every day. It's time to shift your SDS management from a state of reactive chaos to one of proactive control, where every chemical is properly documented and managed from the moment it enters your facility.
Building Your Centralized SDS Command Center
Let’s be honest. Those scattered files, outdated spreadsheets, and dusty binders tucked away in a cabinet aren't just messy—they're a compliance disaster waiting to happen. To get a real handle on safety data sheet compliance, you need to establish a single source of truth for your entire organization. This means ditching the fragmented approach and building a centralized digital library that serves as the command center for all things chemical safety.
The first step is often the toughest: tracking down every single SDS from every corner of your company. Think about it—they could be lurking in R&D lab folders, buried in the procurement team's emails, or sitting on a clipboard at the warehouse receiving dock. Trying to collect all of this manually is a slow, painful process that almost guarantees you'll miss something.
Getting All Your Data in One Place
To build a system you can actually rely on, you need a smart way to get all this information into your library. Modern solutions are all about automating this intake process, which saves a ton of time and cuts down on human error. For instance, some platforms can be set up to monitor a specific email inbox, automatically grabbing new SDS attachments from suppliers and lining them up for processing.
This is where the real magic happens. Instead of a safety manager painstakingly typing data from a PDF into a spreadsheet, AI-powered tools can scan the document and pull out the critical information instantly.
- Chemical Identifiers: CAS numbers, EC numbers, and product names are extracted automatically.
- Hazard Classifications: GHS pictograms, signal words, and hazard statements are identified and logged.
- Revision Dates: The document’s issue date is flagged, so you always know if you're working with the latest version.
- PPE Requirements: Specific recommendations for personal protective equipment from Section 8 are pulled out and made searchable.
This isn't just about creating a digital filing cabinet; it's about transforming static PDFs into structured, usable data. That’s the foundation for a truly intelligent safety program.
The risks of a decentralized or non-compliant approach are real and they escalate quickly, as this flow shows.
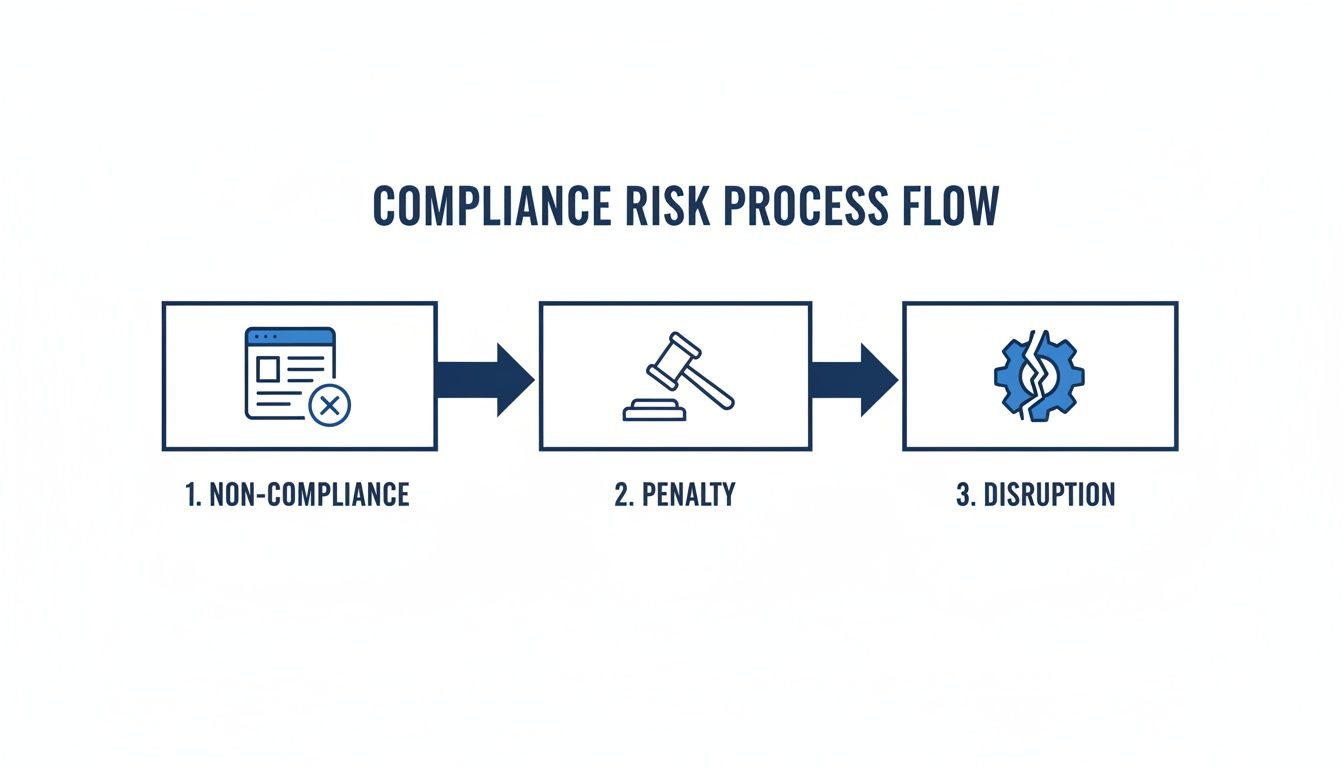
As you can see, a weak foundation doesn't just stay weak—it creates a domino effect of penalties and operational disruptions that can cripple a business.
The Critical First Step: Data Validation
Just having all the documents in one place isn't enough. The integrity of that data is everything. An inaccurate or incomplete SDS is just as dangerous as a missing one, which is why a validation workflow is non-negotiable from day one. You have to ensure the information entering your system is correct and complete.
Your centralized SDS library is only as reliable as the data it contains. If you let unverified or outdated information slip through, you’re building your entire compliance program on a shaky foundation.
This initial effort to confirm data quality pays off massively down the road. It prevents downstream errors in risk assessments, ensures the reports you generate are trustworthy, and turns audit prep from a frantic scramble into a routine check-up.
Validating Supplier Data To Eliminate Risk
An inaccurate supplier SDS is a ticking time bomb in your chemical inventory. Let’s be clear: it's not just a document; it's the bedrock of your entire hazard communication program. When you rely on unverified data, you're accepting a huge liability that puts your workers at risk, compromises safety data sheet compliance, and can bring operations to a grinding halt.
This is exactly why you need a rock-solid validation workflow for every single SDS that comes through the door. This is more than a simple check to see if the document arrived. It's a critical quality control step to ensure the information is accurate, complete, and actually relevant to the regulations you operate under. A mistake on a supplier's end becomes your problem the second that chemical hits your receiving dock. Proactive verification is your best—and frankly, only—line of defense.

Creating Your Quality Control Checklist
A systematic approach is everything. Your team needs to know precisely what to look for so they can spot inconsistencies and red flags quickly. This isn't about memorizing every line of every regulation. It’s about building a practical, repeatable checklist that hits the most critical parts of an SDS.
Here’s a good starting point for your checklist:
- GHS Formatting: Does the SDS have all 16 sections required by GHS? A document missing sections is an immediate fail.
- Revision Date: Is the SDS current? We’ve all seen documents that are years out of date. While reviewing every three years is a common best practice, you must also ensure it reflects the latest regulatory changes.
- Product Identifier Consistency: Does the product name and code on the SDS perfectly match what's on the supplier's label and your purchase order? Even small mismatches can create massive inventory headaches down the line.
- Hazard Classification: Do the hazard statements and pictograms in Section 2 make sense with the chemical composition data you see in Section 3?
- Jurisdictional Requirements: This is a big one. Does the SDS meet the specific requirements for your region, like WHMIS in Canada or CLP in the EU? A lot of companies slip up here.
This can feel like a lot, especially when you're dealing with hundreds of suppliers. For a deeper dive into making this more manageable, check out our guide on SDS data extraction and validation best practices.
Handling Non-Compliant Supplier SDS
So, what do you do when your checklist turns up a problem? This is where your verification workflow really proves its worth. Finding an error—whether it's an outdated SDS or a missing CAS number—should trigger a clear, immediate response.
Let's walk through a real-world scenario. You receive an SDS for a new cleaning solvent. During validation, your system flags a component in Section 3 that’s listed as a Substance of Very High Concern (SVHC) under EU REACH regulations. The problem? The supplier's document makes no mention of this restriction. That’s a critical compliance gap.
A well-defined workflow handles this automatically:
- Flag and Quarantine: The system immediately flags the non-compliant SDS. It also prevents the associated chemical from being fully approved in your inventory management system, essentially putting it on hold.
- Automated Supplier Communication: An email is automatically sent to your supplier contact, detailing the exact issue. For example: "Component with CAS #XXX-XX-X is on the REACH SVHC list, but this is not reflected in Section 15 of the provided SDS." The email requests an updated, compliant document.
- Track Resolution: The system logs the request and sends automated follow-ups until a corrected SDS is received and validated.
By preventing non-compliant chemicals from ever being fully integrated into your facility, you shift from a reactive cleanup model to proactive risk elimination. This protects your operations and ensures you're always audit-ready.
Once you’ve validated the supplier data, the next logical step is to integrate this information into a comprehensive risk register to actively manage potential hazards. If you need a primer, here's an essential guide to risk registers for compliance and safety.
This global patchwork of rules is why manual tracking just isn't sustainable anymore. Despite the GHS's goal for harmony, compliance varies significantly by country. For instance, Canada requires suppliers to update an SDS within 90 days of "significant new data," while the EU's REACH and CLP regulations demand meticulous and immediate updates. With the US HCS mandating revisions by January 19, 2026, it's no surprise that 82% of companies are planning more tech investments for automation, as you can discover more about in this global compliance overview from sdsquantum.com.
Turning SDS Data Into Actionable Safety Workflows
Having a centralized digital library of your Safety Data Sheets is a fantastic start, but let's be honest—its real value isn't unlocked until that data is actively protecting your team on the floor. Static documents collecting digital dust on a server don’t prevent accidents. The goal is to transform that dense information into dynamic, practical workflows that are woven into your daily operations.
This is how safety data sheet compliance moves from a passive record-keeping chore to an active part of your company’s safety culture.
It’s about moving past simple storage. Once you extract the structured data from your SDS—things like pictograms, PPE requirements, and first-aid measures—you can build real-world safety tools that are right there when and where your employees need them.

From Data Points To Daily Protection
The true power of a modern SDS management system is its knack for translating complex, technical information into simple, easy-to-use tools. Instead of an employee having to thumb through a 12-page document during a spill, you can give them immediate, context-specific guidance.
Think about these real-world applications:
- Automated GHS Label Generation: When a chemical gets moved from a large drum into a smaller spray bottle, that secondary container needs a proper label. It's a common and costly compliance miss. With structured data, you can generate a compliant GHS label instantly, complete with the right product identifier, pictograms, and hazard statements. No more guesswork.
- Mobile-Friendly Safety Cards: Imagine placing QR codes on shelves, tanks, or chemical containers. Any employee with a smartphone can scan it and get a quick, one-page summary highlighting the most critical info: required PPE, key hazards, and what to do in an emergency. It’s safety information delivered right at the point of use.
- First Responder Access: In an emergency, every second is critical. A digital system can give first responders instant access to crucial data from Section 4 (First-Aid Measures) and Section 5 (Fire-Fighting Measures) before they even set foot in your facility. That makes for a much safer and more effective response.
The ultimate goal is to make safety information so seamlessly accessible that checking it becomes a natural part of the job, not an inconvenient extra step.
Driving Proactive Risk Management
Beyond just reacting to immediate needs, your centralized SDS data is a goldmine for getting ahead of risks. To build a truly robust safety program, this information has to feed into your broader protocols, like the ones outlined in this practical guide to chemical risk assessment.
When all your chemical information lives in one organized system, you can perform analyses that would be a nightmare with manual methods. This proactive approach lets you answer critical questions before an incident happens.
- Exposure-Based Risk Assessments: You can start combining SDS hazard data with operational details—like how often a chemical is used or the quantities handled—to calculate specific risk scores for different jobs. This is huge. It helps you focus your training efforts and engineering controls on the areas of highest risk.
- Storage Compatibility Management: Section 7 of an SDS gives you clear storage guidance. An automated system can crunch this data from all your SDSs to create a chemical compatibility matrix for your entire inventory. It can flag dangerous combinations and help you design a smarter, safer warehouse layout to prevent accidental reactions.
Manual Vs Automated SDS Workflow Comparison
The difference between a traditional, manual approach and a modern, automated one becomes crystal clear when you put them side-by-side. One is reactive and full of potential human error, while the other is proactive and built for consistency.
| Compliance Task | Manual Approach (Spreadsheets & Binders) | Automated Platform (e.g., NextSDS) |
|---|---|---|
| Secondary Labeling | Manually searching for pictograms and hazard statements, then hoping they are correct. | Instantly generates compliant GHS labels with verified data pulled directly from the SDS. |
| Emergency Info Access | Frantically flipping through a binder to find the right SDS while an incident is unfolding. | QR code scan provides immediate access to first-aid and spill response procedures on a phone. |
| Storage Planning | Relying on tribal knowledge or manually cross-referencing paper SDSs, which is rarely done. | Automatically flags incompatible chemicals, creating a visual map of safe storage areas. |
| Risk Assessment | A time-consuming annual project that often relies on incomplete or outdated information. | A continuous, data-driven process that calculates risk scores and updates them dynamically. |
When you turn your SDS library into the engine that powers these workflows, you fundamentally shift your safety program. It moves from a reactive, paper-based burden to a proactive, data-driven asset. This doesn't just check the box for safety data sheet compliance—it builds a genuinely resilient and safer workplace for everyone.
Staying Ahead with Automated Regulatory Monitoring
Let's be honest: chemical regulations are a moving target. The rules that govern hazardous materials are always in flux, driven by new science and updated government standards. For any safety team, manually trying to keep up with every little change to major regulations like REACH in Europe, TSCA in the U.S., or WHMIS in Canada is a recipe for failure. You're practically guaranteed to miss something important.
This is where bringing in some smart automation changes the game entirely. Instead of relying on industry newsletters and endless website checks, you can put systems in place to do the heavy lifting. It’s a shift that turns the constant scramble for compliance into a managed, confident process.
Moving from Reactive to Proactive Compliance
Think of it like having a regulatory watchdog on duty 24/7. Modern compliance platforms can continuously screen your entire chemical inventory against hundreds of global regulatory lists in real time. This is the key to getting out ahead of changes that could put your business at risk.
The system essentially connects the dots for you. Let's say a new chemical gets added to the REACH Substances of Very High Concern (SVHC) list.
- With a manual process: You'd have to hope someone on your team hears about the update, finds the new list, and then painstakingly cross-checks every single chemical you use against it. It's slow and prone to human error.
- With an automated system: The moment that substance is flagged, you get an alert. The system knows it’s in your inventory and tells you immediately.
That instant notification is a powerful advantage. It gives you time to figure out the impact, talk to your suppliers, and start looking for safer alternatives—long before an auditor shows up at your door.
The real goal of automated monitoring is to catch a potential problem before it ever becomes a violation. It gives your team the intel they need to make smarter, safer decisions on everything from purchasing to daily operations.
There's a reason the market for these solutions is exploding. The global Safety Data Sheet management market is projected to hit $25.4 billion by 2032. Companies are finally waking up to the fact that the cost of non-compliance is just too high. A 2025 survey from PwC found that 82% of executives are planning to invest more in automated monitoring and reporting, which tells you everything you need to know about where the industry is heading. You can dig deeper into this trend by reading the full market analysis on datainsightsmarket.com.
How Automation Keeps Supplier Information Up-to-Date
Automated monitoring isn't just for flagging newly restricted chemicals. It's also critical for making sure your documentation from suppliers is current. When a regulation is updated, manufacturers are often required to issue a new version of their SDS.
But how would you even know they did? Chasing down suppliers is a full-time job in itself.
This is another headache a smart system can solve. Platforms like NextSDS can use AI agents to automatically ping your suppliers on a regular schedule or even scan the web for new documents. To see how this technology is evolving, check out our guide on the future of SDS management with AI and automation.
This completely changes the dynamic of audit prep. When an auditor asks for proof that your SDS library is current, you won't be digging through old emails and call logs. You'll simply pull up a clean, time-stamped history of every supplier check, every communication, and every document update, all handled by the system. What used to be a high-stress scramble becomes a simple, routine check-in.
Answering Your Top SDS Compliance Questions
When you're managing chemical safety, questions are bound to pop up. Getting bogged down in the details of a compliance program is common, so I've put together some straight answers to the questions I hear most often from HSE managers, lab personnel, and procurement teams.
How Often Do Safety Data Sheets Need To Be Updated?
This is a tricky one because there's no single, universal deadline. The real answer depends entirely on the regulations in your specific region, which is precisely why trying to track this manually can quickly become a nightmare.
In the United States, for example, OSHA gives manufacturers three months to update an SDS after they learn of "significant new information" about a chemical's hazards. Canada’s WHMIS rules are similar, requiring updates within 90 days for any "significant new data."
The European Union is even stricter under REACH and CLP regulations, demanding updates "without delay" as soon as new hazard info comes to light. To stay on the safe side, many companies set an internal policy to review their entire SDS library every three years. That's a great habit, but it's not a substitute for the legal requirement to update an SDS the moment new information is available.
An internal three-year review is a good safety net, but it doesn't absolve you of the responsibility to update an SDS the moment a manufacturer issues a revision or a regulation changes. This is where automated systems truly shine, as they can flag aging documents and monitor for supplier updates automatically.
What Is The Difference Between GHS, CLP, And WHMIS?
Think of the GHS (Globally Harmonized System of Classification and Labelling of Chemicals) as the foundational playbook, a set of recommendations from the United Nations. It creates a common, standardized framework for classifying chemical hazards and communicating that information on labels and safety data sheets.
But here’s the key: GHS itself isn’t a law. It’s up to individual countries to adopt its principles and write their own regulations.
- CLP (Classification, Labelling and Packaging) is how the European Union put the GHS into practice.
- WHMIS (Workplace Hazardous Materials Information System) is Canada's version of GHS.
While they share the same GHS DNA, both CLP and WHMIS have their own regional quirks and specific requirements. For any company shipping products internationally, this is a critical detail. An SDS that's compliant in one country might not automatically pass muster in another, which means you often need region-specific documents.
Can I Use A Digital SDS System Instead Of Paper Binders?
Yes, absolutely—and you really should. Regulatory bodies like OSHA are perfectly fine with electronic systems for managing and accessing Safety Data Sheets. There's just one non-negotiable rule: every single employee needs immediate and unimpeded access to the SDS for any chemical they work with, for their entire shift.
So, if you go digital, your system has to be reliable. You'll also need a backup plan for when the power goes out or the network crashes. This could be as simple as a designated laptop with the library saved locally or having a way to print on demand.
Modern platforms make this incredibly easy and far more effective than the old way. Think about it: placing a QR code on a chemical container that links directly to its SDS on a phone is often much faster and more reliable than having someone dig through a massive, and likely outdated, three-ring binder. To learn more about the essential parts of these documents, check out our guide on what a Safety Data Sheet is.
What Are The Most Common SDS Compliance Violations?
Year after year, OSHA's Hazard Communication Standard (HCS) ranks among the most frequently cited standards during workplace inspections. The frustrating part is that most of these violations are straightforward and completely preventable with a good system in place.
The most common slip-ups we see are:
- Missing or Inaccessible SDS: The most basic failure—not having an SDS for every hazardous chemical on site.
- Outdated SDS Documents: Using an old version that doesn’t reflect the latest hazard information or regulatory rules.
- Improper Secondary Container Labeling: Forgetting to properly label smaller containers, like spray bottles, with the required GHS info.
- Inadequate Employee Training: Not teaching employees how to actually find, read, and understand the information in an SDS.
- No Written Hazard Communication Program: Lacking a formal, written plan that details how your company meets HCS requirements.
These common pitfalls almost always trace back to the chaos of manual management. Centralizing your library and automating your workflows is the single most effective way to close these gaps and keep your program audit-ready.
Ready to move beyond spreadsheets and manual tracking? NextSDS offers an all-in-one chemical safety and compliance platform that automates everything from SDS management to real-time regulatory monitoring. Discover how our AI-powered solution can help you build a safer, more compliant workplace by visiting https://nextsds.com.

